B ackground
Patients with relapsed/refractory T-lymphoblastic leukemia/lymphoma (r/r T-ALL/LBL) are clinically manifested by rapid disease progression, poor prognosis and lack of therapeutic options. Currently, only Nailabine was approved by the FDA in 2005 for the treatment of T-ALL/LBL patients who did not respond to at least two chemotherapy regimens or relapsed after treatment, with an ORR of 31% and a CR of 23%. Therefore, there is an urgent need to develop other effective modalities. Apart from its expression in normal T and NK cells and no expression in other tissue cells, CD7 is highly expressed in T-ALL/LBL cells. Therefore, CD7 is considered a potential target for the development of CAR-T therapy for T-ALL/LBL. The challenge, however, is to avoid fratricide caused by the expression of CD7 in T cells. We developed a CAR-T cell injection based on CD7 nano antibodies, named PA3-17 injection, by blocking the expression of CD7 molecule on the surface of T cells through anti-CD7 protein expression blocker (PEBL). After thorough pre-clinical studies demonstrating its safety and efficacy, PA3-17 injection was Investigational New Drug (IND) approved in China in August 2021, and here we report preliminary safety and efficacy data for PA3-17 injection in adult r/r T-ALL/LBL patients in a Phase I clinical study.
M ethodology
The Phase I clinical study (NCT05170568) adopted a classic “3+3” dose escalation schema. T-ALL/LBL patients who met the inclusion/exclusion criteria were deployed by entering three dose groups (DL: 0.5×10 6, 2×10 6, 4×10 6 CAR-T/kg) to evaluate the initial safety, efficacy and dose-limited toxicitys (DLTs). All patients were treated with lymphodepleting chemotherapy pretreatment before CAR-T cell infusion. The primary endpoints were DLTs and maximum tolerable dose (MTD).
R esults
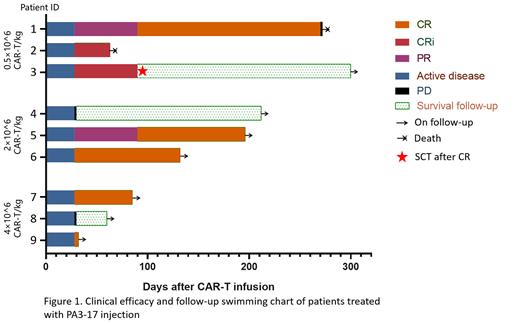
As of June 30 th, 2023, a total of 9 patients were enrolled (3 subjects in each dose group), all of whom received a single infusion of PA3-17 injection and completed a 28-day DLTs assessment. The median age of enrolled patients was 33 years (range 20-64), and 33% (3/9) of patients had previously received hematopoietic stem cell transplantation. The safety analysis showed that 78% (7/9) of patients developed cytokine release syndrome (CRS), of which 33% (3/9) had grade 1-2, 44% (4/9) had grade 3, and no grade 4 CRS occurred, 22% (2/9) of patients experienced 1-2 grade of immune effector cell-associated neurotoxicity syndrome (ICANS), and no grade 3 or higher ICANS occurred. No DLTs occurred in any patients. The efficacy data (Figure 1) showed that the best ORR and CR were both 78% (7/9). The median follow-up time was 137 days, of which 1 patient (Pt1) sustained CR for 9 months until recurrence, 1 patient (Pt3) underwent transplantation after being evaluated as CR in 3 month. One patient (Pt6) had a tumor mass with a diameter greater than 7cm at baseline prior CAR-T infusion but achieved CR 28 days after infusion and is in sustained remission now (Figure 1).
C onclusion
PA3-17 injection has shown a good safety profile and encouraging efficacy in r/r T-ALL/LBL patients, and long-term data are continuing to be collected during follow-up.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal